Lisiraglide CAS 320367-13-3 is a glucagon-like peptide-1 (GLP-1) receptor agonist
Product Name: Lixisenatide
Synonyms: Lixisenatide, >98%;lixisenatide;Lixisenatide|Lixisenatide Acetate;Lixisenatide Acetate;Lixisenatide USP/EP/BP
CAS: 320367-13-3
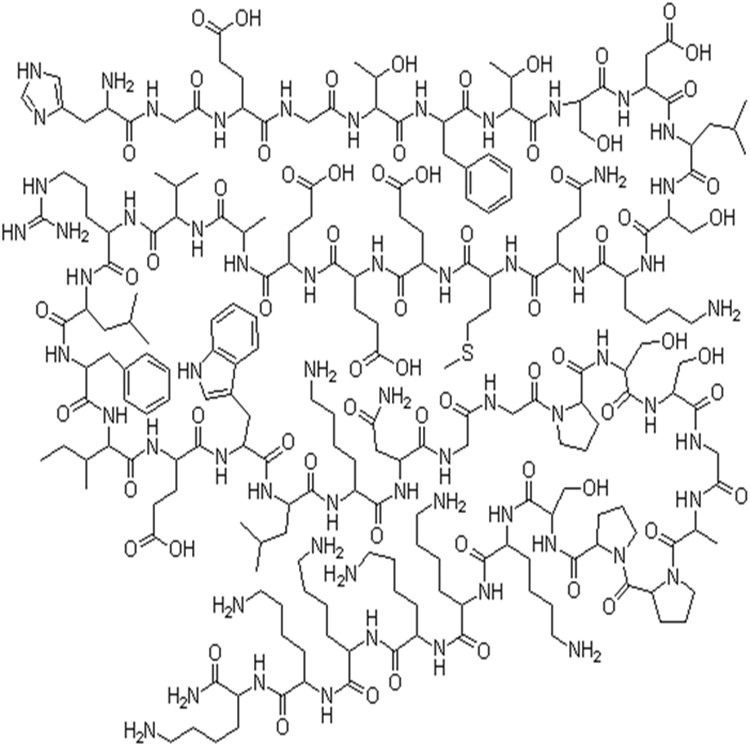
MF: C215H347N61O65S
MW: 4858.53
EINECS:
Product Categories:
Mol File: 320367-13-3.mol
Lisiraglide is a glucagon-like peptide-1 (GLP-1) receptor agonist, a hormone that helps normalize blood sugar levels. The drug’s safety and efficacy were evaluated in 10 clinical trials that enrolled a total of 5,400 patients with type 2 diabetes. In these trials, lisiramide was evaluated both as monotherapy and in combination with other FDA-approved diabetes medications, including metformin, sulfonylureas, pioglitazone and basal insulin. In these trials, the use of lisiramide improved glycated hemoglobin (an indicator of blood glucose levels) levels. In addition, more than 6,000 patients with type 2 diabetes at risk for atherosclerotic cardiovascular disease were treated with lisiramide or placebo in the Cardiovascular Outcomes Trial. The use of lisiramide in these patients did not increase the risk of adverse cardiovascular events. Lisiragide should not be used to treat patients with type 1 diabetes or patients with increased ketones or urine in the blood (diabetic ketoacidosis). The most common side effects associated with lisiramide are nausea, vomiting, headache, diarrhea, and dizziness. Hypoglycemia is another common side effect in patients treated with lisiramide and other antidiabetic drugs such as sulfonylureas and/or basal crop. In addition, severe hypersensitivity reactions (including hypersensitivity) have been reported in clinical trials with Lisiragib.
Lixisenatide is a drug currently used to treat diabetes and is also a GLP-1 receptor agonist. Lixisenatide (Lyxumia) was approved for marketing in Europe in 2013 and is an injectable diabetic treatment based on Lixisenatide, which is administered once a day, together with other medications or insulin, for patients whose blood glucose levels can only be controlled by medications.
Note of Lisiraglide CAS 320367-13-3
For research use only, not for human use





Reviews
There are no reviews yet.