Carbetocin Acetate CAS 37025-55-1 Prevention of weak uterine contractions and postpartum hemorrhage
Product Name: CARBETOCIN
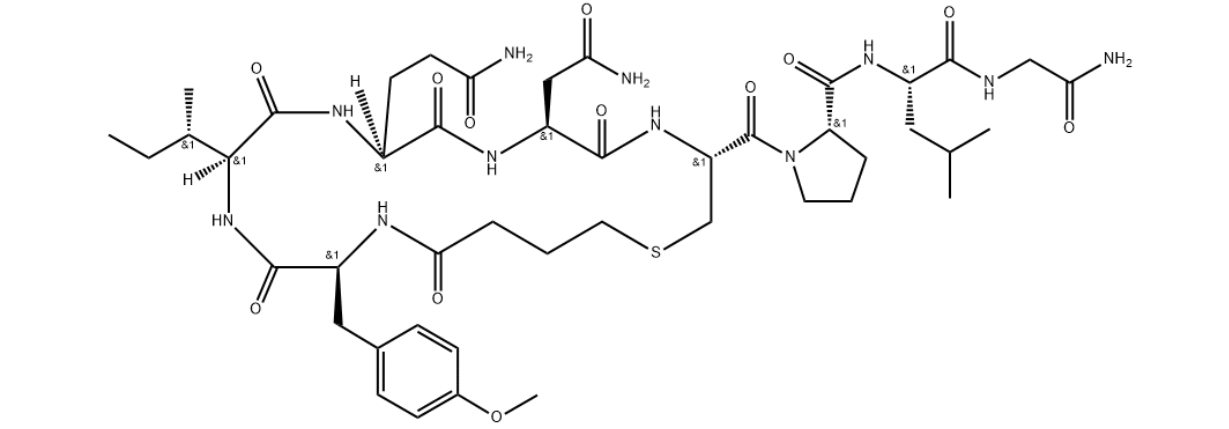
Synonyms: BUTYRYL-TYR(ME)-ILE-GLN-ASN-CYS-PRO-LEU-GLY-NH2, (SULFIDE BOND BETWEEN BUTYRYL-4-YL AND CYS);BUTYRYL-TYR(ME)-ILE-GLN-ASN-CYS-PRO-LEU-GLY-NH2 TRIFLUOROACETATE SALT;(BUTYRYL1,TYR(ME)2)-1-CARBAOXYTOCIN TRIFLUOROACETATE SALT;(BUTYRYL1,TYR(ME)2)-OXYTOCIN;(BUTYRYL1,TYR(ME)2)-OXYTOCIN TRIFLUOROACETATE SALT;CARBETOCIN;CARBETOCIN TRIFLUOROACETATE SALT;(2-O-METHYLTYROSINE)-DE-AMINO-1-CARBAOXYTOCIN
CAS: 37025-55-1
MF: C45H69N11O12S
MW: 988.17
EINECS: 253-312-6
Product Categories: Other APIs;Peptide;Vasopressin and Oxytocin receptor;Intermediates & Fine Chemicals;Pharmaceuticals
Mol File: 37025-55-1.mol
Carbetocin is a synthetic long-acting oxytocin 8-peptide analogue with agonist properties, with clinical and pharmacological properties similar to those of naturally occurring oxytocin. Like oxytocin, carbetocin binds to the oxytocin receptors in the smooth muscle of the uterus, causing a rhythmic contraction of the uterus that increases its frequency and increases uterine tone on top of the original Chemicalbook contraction. In the non-pregnant state, the level of oxytocin receptors in the uterus is low, increasing during pregnancy and peaking at delivery. Therefore, cabergoline has no effect on the non-pregnant uterus, but has an effective uterine contraction effect on the pregnant uterus and the uterus that has just given birth.
CARBETOCIN Chemical Properties of Carbetocin
alpha D -69.0° (c = 0.25 in 1M acetic acid)
Boiling point 1477.9±65.0 °C(Predicted)
density 1.218±0.06 g/cm3(Predicted)
storage temp. -15°C
form powder
pka 13.07±0.70(Predicted)
color white to beige
Function and Application of Carbetocin
For use after elective epidural or lumbar anesthesia for cesarean delivery to prevent weak uterine contractions and postpartum hemorrhage.
Caesarean section under other anesthesia for emergency caesarean section, classic caesarean section, epidural or spinal anesthesia, or maternal presence with significant heart disease, history of hypertension, known coagulopathy or liver, kidney and endocrine diseases (excluding gestational diabetes) The use of carbetocin has not been studied. The administration of carbetocin after vaginal delivery has not been properly studied and the dose has not been determined.





Reviews
There are no reviews yet.